In clinical research, a well-designed study is crucial to support the study objectives and answer the specific research questions. The design of a clinical study includes the various treatment arms, the duration of treatment, and the sequential flow of various study parts. In this article, the different types of clinical study design are discussed.
Overview of Study Designs
There are two major designs of clinical studies: observational and experimental. The experimental or interventional studies evaluate the effect of a particular intervention or treatment on participants or groups of people. The observational or non-interventional studies observe an existing situation without assigning participants to a specific intervention or treatment. A diagram of the different experimental and observational studies is presented below.
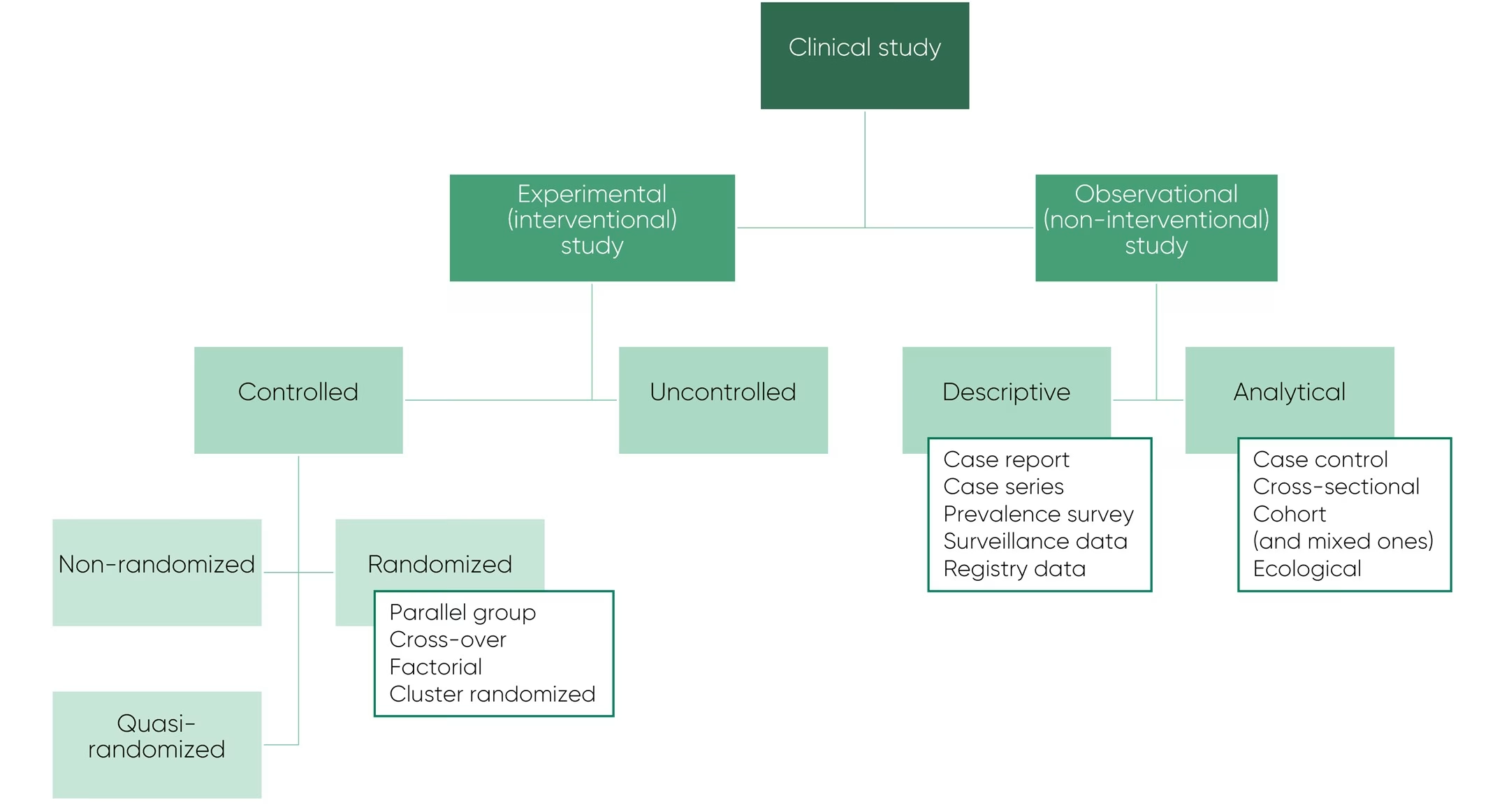
Experimental Study Designs
Experimental studies are subdivided in the uncontrolled and controlled trials. Uncontrolled trials do not include a control group against which a certain treatment is compared. They are performed as a precursor to controlled trials to assess the appropriate treatment method, the estimated effect size, the most suitable patient population to investigate the efficacy, and the likelihood of a clinical effect. However, uncontrolled trials investigate only a single intervention and due to multiple possible causes of the effect, a true causal relationship cannot be established.
Controlled trials compare a treatment to a certain control group. There are several options for this control group depending on the study objectives and regulatory, operational, and ethical considerations:
- Placebo: a formulation without an active pharmaceutical ingredient that is indistinguishable from the test treatment regarding packaging, administration, taste, smell…
- Current standard of care: to compare whether the active treatment is better than the existing clinical practice.
- Active comparator: to assess whether the active treatment is superior to the comparator.
In controlled trials, different methods can be used to allocate participants to study groups. Non-randomized allocation introduces bias into the study design and leads to more variability in the observed effects. Quasi-randomized involves allocation of participants to groups using a method that is not truly random, eg, alternating the allocation to arm A or arm B. However, this introduces selection bias leading to systematic differences between the groups. Randomization ensures equal distribution of participants between the different groups and can vary from simple randomization to highly complex computerized web-based systems:
- Simple randomization uses a single randomization sequence such as a coin toss. Participants are randomly allocated to groups based on a constant probability. However, this may create an imbalance in the number of participants allocated to each group.
- Block randomization uses equal sized blocks that are assigned with all possible treatment sequences and picked randomly to generate the randomization sequence. This will lead to a balance between the different groups but will still include a component of predictability.
- Stratified randomization randomizes participants by key baseline demographics to equally distribute these characteristics between groups. This ensures compatibility between the different groups.
Randomized, controlled trials are the current golden standard for experimental studies because they provide the most robust and reliable results. They can be designed in different ways depending on the objectives:
- In a parallel group design, participants are randomized to a specific group and keep the same treatment until trial completion or discontinuation. The simplest parallel group design includes 1 intervention and 1 control arm, but the design can also involve more than 2 arms.
- In a cross-over design, participants receive all treatments in a randomized sequence with evaluation of outcomes and a wash-out period in between administration of each treatment.
- Factorial designs compare multiple treatments given as mono- or combination therapy, creating a group for each possible combination.
- In cluster randomized trials, participants are grouped into clusters which are then randomized to the different treatment groups. These clusters can vary in size, ranging from families, school classes or schools, villages, or administrative areas.
Observational Study Designs
Observational studies are subdivided into descriptive and analytical studies. Descriptive studies describe characteristics and distribution of a disease by person, place, or time. There are different types of descriptive studies:
- A case report presents patients in their natural clinical setting. A case series is when there is more than one case report. Case reports or series can be useful to steer further research by generating new research questions, but they cannot be used to generate conclusions since no associations can be investigated.
- Prevalence surveys, surveillance data, and registry data are similar to each other, but differ in how the data were collected: from surveys, surveillance trials, or existing registries.
Analytical studies try to establish an association between exposure and outcome such as the cause of disease or a link between a biomarker and the chance of treatment success. There are multiple types of analytical studies:
- Case-control studies start with a specific outcome and try to identify which prior exposures might be associated with the development of the outcome.
- Cohort studies start with a certain exposure and participants are followed up to determine whether a specific outcome occurs. Cohort studies look at the incidence of a disease rather than the prevalence.
- Cross-sectional studies represent more a snapshot in time where both exposure and outcome and other information is measured at the same time. These studies look at the prevalence of a disease rather than the incidence.
- Ecological studies are performed to study the relation between exposure and outcome or disease at the level of a specific group rather than the individual.
However, there is no single perfect study design to answer a given research question. Each study design is accompanied with advantages and disadvantages. The choice of study design depends on several factors, such as the research question, timeframe, budget, access to sources of data, and ethical considerations. For more details on the clinical study designs, please refer to the training “Overview of Study Types and Designs” available on our e-learning platform Emtex Academy. This comprehensive training consists of 2 modules and covers all kinds of clinical study types and designs including examples, features, advantages, and disadvantages of each study type.
The Medical Writer can be involved in the document development process (eg, write with team input, circulate for review, implement review comments, facilitate meetings, ensure consistency) and can indicate issues or make useful suggestions. More information about the Clinical Trial Protocol?
